Generic Drug Cost Calculator
Compare Your Prescription Costs
See how much you could save by switching to generic medications. Based on FDA-approved generics with therapeutic equivalence.
Important note: This calculator shows potential savings based on typical price differences. FDA-approved generics have identical active ingredients and bioequivalence. For drugs with narrow therapeutic index (like warfarin or levothyroxine), consult your doctor before switching.
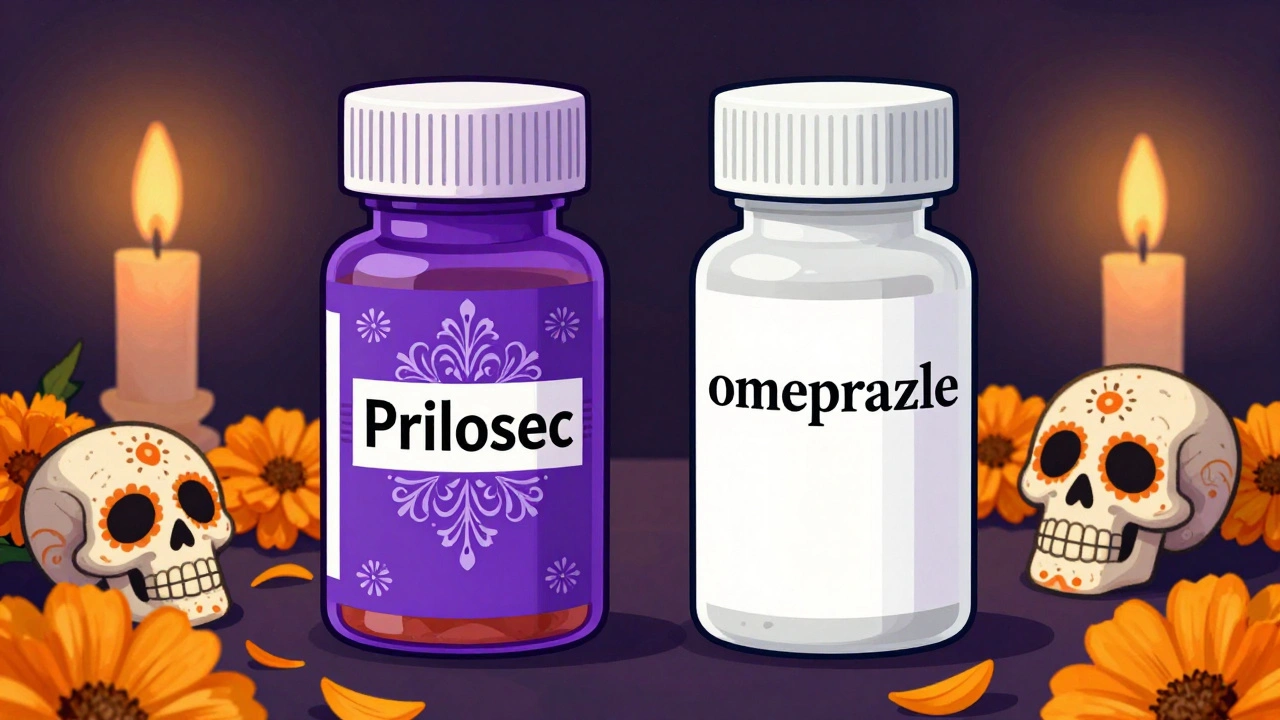
When you pick up a prescription, you might see two very different bottles on the counter. One says Prilosec, costs $150, and has bright purple capsules. The other says omeprazole, costs $4, and is a plain white pill. You might wonder: Is this cheaper version any good? Does it even work the same?
The short answer is yes - and the FDA makes sure of it. Generic drugs aren’t cheap knockoffs. They’re exact chemical twins to brand-name drugs, down to the active ingredient. The only real differences are in the label, the color, and the price.
What’s Actually in the Pill?
Every pill, whether it’s branded or generic, has two kinds of ingredients: active and inactive. The active ingredient is what actually treats your condition. For example, the active ingredient in Lipitor is atorvastatin. The same active ingredient is in every generic version of Lipitor. The FDA requires that generics contain the exact same amount of that ingredient, in the same form, and deliver it the same way - whether it’s swallowed, injected, or absorbed through the skin.
What changes are the inactive ingredients: the fillers, dyes, binders, and coatings. These don’t affect how the drug works. They’re there to hold the pill together, make it easier to swallow, or just make it look different. That’s why a generic version of a blue pill might be yellow. U.S. trademark law forces this. If generics looked exactly like the brand, they’d be breaking patent rules - not medical ones.
How the FDA Makes Sure Generics Work the Same
The FDA doesn’t just take a manufacturer’s word for it. Before a generic drug hits the shelf, it must pass a strict test called bioequivalence. This means scientists give the generic and the brand-name drug to healthy volunteers and measure how quickly and completely the body absorbs the medicine. The generic’s absorption rate must fall within 80% to 125% of the brand’s. That’s not a wide margin - it’s tighter than the natural variation you’d see between two different batches of the same brand-name drug.
That’s not theory. It’s data. A 2021 study in JAMA Internal Medicine tracked over 2 million patients using generic and brand-name cardiovascular drugs. No difference in heart attacks, strokes, or deaths. Another review in BMJ Open looked at 47 clinical trials and found no evidence that generics were less effective.
The FDA’s Orange Book lists every approved generic and its therapeutic rating. Look for an “A” rating - that means it’s approved as therapeutically equivalent. If you’re switching from one generic to another, the rating stays the same. But if you’re switching from brand to generic, or between two different generics, your doctor might want to check your blood levels - especially for drugs like warfarin, levothyroxine, or phenytoin. These have a narrow therapeutic index. A tiny change in blood concentration can make a difference. That’s not because generics are weak. It’s because the medicine itself is powerful and precise.
Why the Label Looks So Different
The brand-name label says “Lipitor.” The generic label says “atorvastatin.” That’s not a trick. It’s the law. Brand names are trademarks. Generic labels must use the drug’s chemical name. But everything else? The warnings, side effects, dosing instructions, contraindications - all of it must match exactly. The FDA’s 2021 guidance requires that generic labeling be identical in content, wording, and format to the brand’s. You’re not missing out on safety info.
Some patients get confused when their pill changes color or shape. A University of Michigan study found that 12% of people hesitated when they got a new-looking pill - even if it was the same drug. That’s not about effectiveness. It’s about familiarity. If you’ve been taking a white oval pill for years and suddenly get a blue round one, your brain says, “Something’s wrong.” But your body doesn’t care. It only cares about the active ingredient.

Cost Difference Isn’t a Trick - It’s the System Working
Brand-name drugs cost so much because the company paid for research, clinical trials, marketing, and patent protection. That’s expensive. Once the patent expires, any company can make the drug. No need to repeat the trials. That’s why generics cost 80% to 85% less. At Walmart, a month’s supply of generic atorvastatin is $4. The brand? $375. That’s not a scam. That’s competition.
From 2007 to 2016, generic drugs saved the U.S. healthcare system $1.67 trillion. In 2023 alone, they saved $313 billion. And the FDA says 90% of generics cost less than $10 a month. That’s why so many people can afford their meds. A Kaiser Permanente survey found 78% of patients said generics helped them stick to their treatment plan. Skipping doses because of cost? That’s when health gets risky.
When Generics Might Need Extra Care
For most drugs, switching is safe and simple. But some drugs need more attention. Drugs like levothyroxine (for thyroid), warfarin (for blood thinning), and phenytoin (for seizures) are sensitive. Small changes in blood levels can cause problems. That’s not because generics are inferior. It’s because these drugs have a very narrow window between working and causing harm.
Pharmacists and doctors know this. If you’re on one of these drugs, your provider might check your blood levels after switching - just to be sure. That’s standard practice. It’s not a red flag. It’s good medicine.
Complex drugs like insulin, biologics, and auto-injectors (like EpiPens) are harder to copy. The FDA calls these “complex generics.” They’re not simple pills. They’re systems. That’s why there are fewer generic versions. But progress is happening. In September 2023, the FDA approved the first generic version of semaglutide (Ozempic). More complex generics are coming. The FDA’s 2024-2028 plan aims to cut review times for these drugs by 20%.

What You Can Do
If your doctor prescribes a brand-name drug, ask: “Is there a generic?” Most of the time, the answer is yes. Your pharmacist can tell you. If your insurance covers generics, you’ll save money automatically - even if you don’t ask.
Don’t be afraid to switch. Thousands of patients do it every day without issue. If you notice something strange - like new side effects after switching - talk to your doctor or pharmacist. But don’t assume it’s the generic’s fault. It’s more likely your body adjusting, or a coincidence.
Check the FDA’s Orange Book online. It’s free. Look up your drug. See if it has an “A” rating. If it does, you’re good to go.
And if your pill looks different? Don’t panic. It’s probably the same medicine - just made by a different company. The FDA requires that change. It’s not a sign of lower quality. It’s proof that the system is working.
Who Makes These Drugs?
Generic drugs aren’t made in back-alley labs. They’re made by big, regulated companies like Teva, Sandoz, and Amneal. Teva alone makes about 1 in 7 generic pills in the U.S. These companies are held to the same standards as Pfizer or Merck. Their factories are inspected by the FDA. Their quality control is just as strict. In fact, many brand-name companies also make their own generics after their patent expires.
The U.S. generic drug market was worth $127 billion in 2022. It’s growing. And with over $268 billion in brand-name drugs set to lose patent protection by 2028, more generics are coming. The system is designed to work this way - to save money without sacrificing safety.
Final Thought: It’s Not About the Label - It’s About the Medicine
Generic drugs aren’t second-rate. They’re second-generation. They’re the result of science, regulation, and competition. The label might say something different. The color might be wrong. But the medicine? It’s the same. The FDA doesn’t approve generics because they’re cheap. They approve them because they work.
If you’re worried, ask your pharmacist. They see this every day. They’ll tell you: 90% of prescriptions filled in the U.S. are generics. And for good reason. They save lives - not just money.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for quality, safety, strength, purity, and performance as brand-name drugs. Every generic must prove it delivers the same active ingredient in the same way and at the same rate. Post-market monitoring through the FDA’s Adverse Event Reporting System tracks any issues - and the data shows no difference in safety.
Why do generic pills look different?
U.S. trademark laws require generic drugs to look different from brand-name versions. That means different color, shape, size, or markings. These changes are purely cosmetic and have zero effect on how the drug works. The active ingredient and its delivery are identical. The difference is only in appearance - not effectiveness.
Can I switch between different generic brands?
Yes, for most drugs. If a generic has an “A” rating in the FDA’s Orange Book, it’s considered therapeutically equivalent to the brand and to other generics with the same rating. But for narrow therapeutic index drugs like warfarin or levothyroxine, your doctor may prefer you stick with one manufacturer to avoid small variations in absorption. Always check with your pharmacist before switching between generic brands.
Do generics take longer to work?
No. Bioequivalence testing ensures that generics are absorbed into the bloodstream at the same rate and to the same extent as the brand-name drug. Studies show no meaningful difference in how quickly generics start working. If you feel a delay, it’s likely due to other factors - like diet, other medications, or your body’s natural variation - not the generic itself.
Why are some drugs still only available as brand-name?
Some drugs are complex - like biologics, insulin, or auto-injectors - and harder to copy exactly. These require advanced manufacturing and testing. The FDA calls them “complex generics.” While the first generic versions of drugs like semaglutide (Ozempic) were approved in 2023, many others are still in development. Patent protections and technical challenges delay availability, but the trend is moving toward more generics, especially as patents expire.